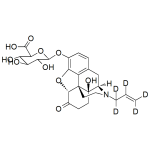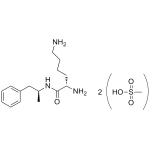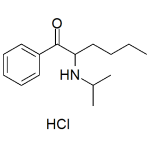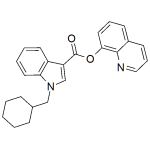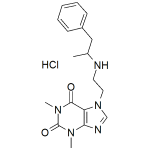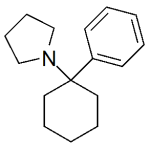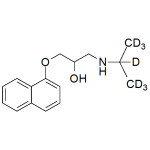Chemtos
Service, Quality, Speed
Chemtos
Service, Quality, Speed
Chemtos

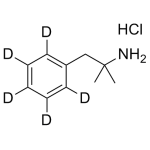
Phentermine-d5 HCl 0.1mg/ml
High purity Phentermine labeled d5 HCl solution includes a comprehensive Certificate of Analysis and all supporting analytical data
Naloxone-3-beta-D-glucuronide-d5
High purity Naloxone-3ß-D-Glucuronide labeled-d5 solution includes a comprehensive Certificate of Analysis and all supporting analytical data
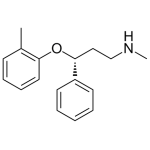
Atomoxetine 1mg/ml
High purity Atomoxetine solution includes a comprehensive Certificate of Analysis and all supporting analytical data
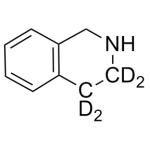
Tetrahydoisoquinoline Labeled d4
High purity Tetrahydoisoquinoline Labeled d4 includes a comprehensive Certificate of Analysis and all supporting analytical data
Lisdexamfetamine Dimesylate
High purity Lisdexamfetamine Dimesylate includes a comprehensive Certificate of Analysis and all supporting analytical data
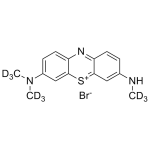
Azure B Bromide labeled d9
High purity Azure B Bromide labeled d9 includes a comprehensive Certificate of Analysis and all supporting analytical data
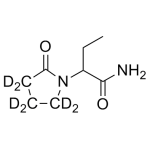
Levetiracetam-d6 (rac) 1mg/ml
High purity Etiracetam-d6 solution includes a comprehensive Certificate of Analysis and all supporting analytical data
N-Isopropylhexedrone HCl (NiPH)
High purity N-Isopropylhexedrone HCl (NiPH HCl, 2-(Isopropylamino)-hexanophenone, N-Isopropylhexanophenone, Alpha-Isopropylaminohexanophenone ) includes a comprehensive Certificate of Analysis and all supporting analytical data
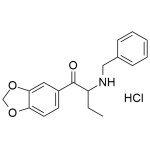
Benzyl-Butylone HCl (BMDB)
High purity Benzyl-Butylone HCl (BMDB HCl, 1-(1,3-benzodioxol-5-yl)-2-(benzylamino)butan-1-one) includes a comprehensive Certificate of Analysis and all supporting analytical data
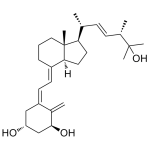
Ercalcitriol (1alpha,25-dihydroxyvitamin D2)
High purity 1alpha,25-dihydroxyvitamin D2 (Ercalcitriol) includes a comprehensive Certificate of Analysis and all supporting analytical data
BB-22, QUCHIC
High purity BB-22, QUCHIC (SGT-32 or 1-(cyclohexylmethyl)-1H-indole-3-carboxylic acid 8-quinolinyl ester) includes a comprehensive Certificate of Analysis and all supporting analytical data
Fenethylline HCl 1mg/ml
High purity Fenethylline HCl solution includes a comprehensive Certificate of Analysis and all supporting analytical data
PCPy (Rolicyclidine, 1-Phenylcyclohexylpyrrolidine)
High purity PCPy (Rolicyclidine, 1-Phenylcyclohexylpyrrolidine) includes a comprehensive Certificate of Analysis and all supporting analytical data
Propranolol-d7 0.1mg/ml
High purity Propranolol labeled-d7 solution includes a comprehensive Certificate of Analysis and all supporting analytical data
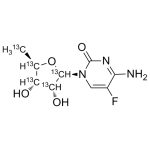
5'-Deoxy-5-fluorocytidine Labeled 13C5
High purity 5'-Deoxy-5-flurocytidine Labeled 13C5 includes a comprehensive Certificate of Analysis and all supporting analytical data